Health On The Net Foundation
Health On the Net Foundation’s origins go back to September 7-8, 1995, when some of the world’s foremost experts on telemedicine gathered in Geneva, Switzerland, for a conference entitled “The Use of the Internet and World-Wide Web for Telematics in Healthcare” .
The 60 participants came from 11 countries. They included U.S. heart surgeon Dr Michael DeBakey, physicians and professors, researchers and senior representatives of the World Health Organisation (WHO), International Telecommunication Union (ITU), the European Laboratory for Particle Physics (CERN), the European Commission, the National Library of Medicine and the G7-Global Healthcare Applications Project.
As the conference wound up, they unanimously voted to create a permanent body that would, in the words of the programme, “promote the effective and reliable use of the new technologies for telemedicine in healthcare around the world. HON’s site went live some six months later. On March 20, 1996, www.hon.ch became one of the very first URLs to guide both lay users and medical professionals to reliable sources of healthcare information in cyberspace.”
Health On The Net Code Of Conduct and DAVAL / Nevasic compliance.
1. Authoritative Information
Term: Any medical or health advice provided and hosted on this site will only be given by medically trained and qualified professionals unless a clear statement is made that a piece of advice offered is from a non-medically qualified individual or organisation.
DAVAL Compliance:
Other than where clearly stated, information displayed on this site is provided by a non-medically qualified organisation and in general provided by non-medically qualified members of our staff and board. Where information is provided by medical professionals we include their name and qualifications and if appropriate their contact points.
Our board of editors and contributors are;
David Gosley – Not medically trained
Jo Harris – Not medically trained
Alasdair Hirst – Not medically trained
2. The purpose of this web site
Term: The reason for the web site.
Our mission is to help the on-line community to gain access to considered and useful information that may help them while they are seeking information about the conditions and causes of nausea and vomiting.
This site is here to help the on-line community to understand more fully the general information available regarding the issues and problems and causes of nausea and vomiting.
It is not intended to be a sole source and it is not to be considered the only source and it is not to be regarded as a definitive on the world wide stage of the study of nausea and vomiting.
- This site does not offer consultation in regard to any medical condition and staff of the site company do not respond to medical or health related questions which by their nature should be directed to your own personal health expert / GP / physician or other specific qualified health advisor.
- The information provided on this site is designed to support, not replace, the relationship that exists between a patient/site visitor and his/her existing physician or other medical advisor.
- In being here this site also offers confirmed evidence supporting the clinical trials and results of those trials using a product named “nevasic”. The site is here to bring together sufficient information as to allow a visitor to draw their own informed conclusion as to whether they would like to purchase a product shown to have beneficial properties in dealing with the symptoms of nausea and vomiting.
- As this site has a commercial aspect it does not contain information about alternatives methods for dealing with the symptoms of nausea and vomiting.
3. Confidentiality & Privacy
Term: Confidentiality of data relating to individual patients and visitors to a medical/health Web site, including their identity, is respected by this Web site. The Web site owners undertake to honour or exceed the legal requirements of medical/health information privacy that apply in the country and state where the Web site and mirror sites are located.
DAVAL Compliance:
Due to the nature of our web site and the fact that many who visit our web site are seeking information of a personal health nature we do not collect or store any information that could be used by anyone to identify and person or organisation.
We do not collect data about any site visitors, and we do not employ any cookie technology.
While this type of data is regarded by many as important to how their visitors respond to comments and links on pages and use the information to track page paths etc – we do not.
“Our Privacy Policy”
- We do not collect personal or identifiable information about the visitors to this site.
- We do not use cookies to collect personal or identifiable information about the visitors to this site.
- We do not display patient information, with the exception of authorised testimonial.
4. Attribution
Term: Where appropriate, information contained on a site will be supported by clear references to source data and, where possible, have specific HTML links to that data. The date when a clinical page was last modified will be clearly displayed (e.g. at the bottom of the page).
DAVAL Compliance:
Data from third party sources is clearly marked, with links to those sources.
5. Justifiability
Term: Any claims relating to the benefits/performance of a specific treatment, commercial product or service will be supported by appropriate, balanced evidence in the manner outlined above in Principle 4.
DAVAL Compliance:
Any and all statements relating to the benefits / performance of Nevasic® are based on facts derived from the direct result of research and clinical trials run by authoritative third party organisations and research bodies using Nevasic®. While there are statements made throughout this site and we have pages dedicated to specific niche areas we feel that you will appreciate the transparency and speed of reference offered by placing the research abstracts here;
Motion or “Travel” Sickness
In the case of statements made in relation to Travel Sickness:
Research in the form of a clinical trial carried out by Westminster College of Medicine;
Journal of Travel Medicine – Volume: 10 • Issue: 02 • 2003 • March • Page: 108
Behavioural Methods of Alleviating Motion Sickness: Effectiveness of Controlled Breathing and a Music Audiotape:
Fleur D.Yen Pik Sang, Jessica P. Billar, John F. Golding, and Michael A. Gresty, Jessica P. Billar, John F. Golding: Department of Psychology, University of Westminster, London; Fleur D. Yen Pik Sang, Michael A. Gresty: MRC Spatial Disorientation Group, Imperial College School of Medicine, London, United Kingdom.
ABSTRACT
Background: Behavioural countermeasures for motion sickness would be advantageous because of the side effects of antiemetic drugs, but few alternative treatments are available. The objective of this study was to compare the effectiveness of controlling breathing and listening to a music audiotape designed to reduce motion sickness symptoms, on increasing tolerance to motion-induced nausea.
Method: Twenty-four healthy subjects were exposed to nauseogenic Coriolis stimulation on a rotating turntable under three conditions: whilst focusing on controlling breathing; listening to a music audiotape; or without intervention (control). The three conditions were performed by each subject according to a replicated factorial design at 1-week intervals at the same time of day. Ratings of motion sickness were obtained every 30 seconds. Once a level of mild nausea was reached subjects commenced controlling breathing or listened to the music audiotape. Motion was stopped after the onset of moderate nausea.
Results: Mean (± SD) motion exposure time in minutes tolerated before the onset of moderate nausea was significantly longer (p < .01) for controlling breathing (10.7 ± 5.6 min) and longer (p < .01) for music (10.4 ± 5.6 min) compared with control (9.2 ± 5.9 min).
Conclusions: Both controlling breathing and the music audiotape provided significant protection against motion sickness and with similar effectiveness. These nonpharmacologic countermeasures are only half as effective as standard doses of antimotion sickness drugs, such as oral scopolamine; however, they are easy to implement and free of side effects.
Morning Sickness or “NVP”
In the case of statements made in relation to Nevasic® and its use for assistance in dealing with the symptoms of nausea and vomiting in connection with nausea and vomiting of pregnancy (NVP) or “morning Sickness”.
The research was commissioned by:
The Winchester and Eastleigh NHS Trust in conjunction with mothers attending the Andover NHS Birth Centre.
Organised by:
CHAIR OF DEANERY SPECIALIST TRAINING COMMITTEE
Mr M J Heard FRCOG
Department of Obstetrics and Gynaecology
Royal Hampshire County Hospital
Romsey Road
Winchester
SO22 5DG
The study was managed by:
Mrs. Lynne Mayo, SRN, SCM.
The full report generated by the research was published in “The Practising Midwife”;
The Study Of MorningWell™
(MorningWell was the brand name at the time of research and is now re-branded Nevasic®)
Lynne Mayo SRN/SCM
A sound remedy?A new treatment for ‘Morning Sickness’
Lynne Mayo and her colleagues at the Andover Birth Centre were sceptical about the benefits of an audio tape – but now they’re impressed.Lynne Mayo SRN. SCM. is a Community and Birth Centre Midwife based at Andover Birth Centre.
HOW OFTEN DO WE SEE the elation of pregnancy diminished by the distress of “morning sickness” Nausea and vomiting in pregnancy (NVP) is non-specific, it affects approx. 80% of pregnant women[1], and the range of symptoms extend from mild nausea through to the extreme of Hyperemesis Gravidarum[2]. It can last all day, it can keep recurring in waves. The actual aetiology is still unclear and there are various theories but NVP is mainly attributed to altered hormone function, physiological and metabolic changes and genetic incompatibility [3]. This preliminary study introduces the ‘Morning Well tape’ in to the lives of pregnant women and exciting initial findings have shown a marked reduction of symptoms.
“I can’t eat anything” “I feel wretched” “I’m nauseated all the time” “I can’t cope with the family” “I can’t get out of bed and I’ve never been ill in my life” I’m struggling to be at work” etc. These words are so familiar, and what is our response? Sympathy. Whitehead SA, Andrews PLR, Chamberlain GVP 2. Lacroix R, Easton E, Melzack R. Nausea and vomiting during pregnancy 3. Lindsay P. Vomiting.In: Sweet B. Mayes Midwifery, 12th edition. London: Bailliere Tindall,1997The MorningWell tape was derived from TravelWell.
TravelWell is a successful travel sickness product, endorsed by Ellen McArthur. It was developed over a number of years, working on the physiology of emesis; there is a recognized correlation between the brain, the vestibular system, and the gut.
[4] The audio tape was devised to react on the vestibular system in such a way that it interrupted the emesis signals to the gut and stopped the sickness. The frequencies and pulses are bordering on our recognisable audio spectrum, but still have a profound effect on the ear. The music overlaying the sounds just makes for more pleasurable listening. It has to be used with a personal stereo. DAVAL, the Andover company responsible, had never thought of the tape being used for anything other than motion sickness, until a couple of women contacted them to ask if it would help in pregnancy, as their symptoms were just the same. DAVAL made no claims at all, but the women used the tape and it worked. Fascinated by this result, DAVAL came to us. It was radical, it was fascinating, and we were cynical!
We looked at the product in depth with safety, the overriding priority. Safety for the woman and the fetus. The tape was non- invasive and under the control of the woman (turned on and off at will). We undertook this preliminary study to find out if this tape worked? In order to do this, we needed to ask the women, and we needed to contact them early enough to offer the tape. Together with DAVAL we devised a questionnaire, which was subsequently passed by the Ethics Committee.
QUESTIONNAIRE FORMAT
We advertised in GP Surgeries, the local paper, local radio, NCT magazine, that we were running a preliminary study on MorningWell – not that we were endorsing it – but that we were interested in its efficacy. We put a brochure into every booking pack and the midwives offered a free tape if NVP was a problem.
We were all very sceptical, but keen to get the responses. 4. Veyrat-Follet C; Farinotti R; Palmer JL.. Physiology of Chemotherapy-induced emesis and antiemetic therapy. Medline Indexing Date :199706. www.medscape.com/medline.
Nevasic fatured in the Practical Midwife Journal
As published in:
The Practising Midwife – vol: , No 10: November 2001
This is the one safe option that a midwife can give. We can support and we can acknowledge their distress. As midwives we can recommend ginger, dry biscuits, frequent snacks, Travel bands, and various other helpful hints. All have anecdotal efficacy. Homeopathic, herbal remedies and acupuncture, can only be prescribed by a specialist. There is no evidence based cure for NVP.
The historical evidence with systemic medication (thalidomide) precludes women from wanting to take anything at all, and NVP is a condition that women expect and tolerate.
When the DAVAL Company approached the Andover Birth Centre, late last year, to ask if we would be interested in an audio-tape that might ease morning sickness—we were intrigued. This was a completely new and radical idea. Is it a relaxation tape? Is it subliminal messages—“You will not be sick!” —Is it just pleasant music to take your mind off the nausea? We wanted to know more.
ANALYSIS & RESULTS
The early feedback from the questionnaires, though positive, showed that there were some difficulties understanding the use of the tape. The programme is made up of 3 phases. The first draws the vestibular system away from the general acoustic and balancing priority, the second is focused on preventing the transmission of the emetic signals to the brain and the gut, and the last phase returns the vestibular system to normal operation. (There have been no reports of any problems with balance). The instructions now highlight the necessity of rewinding the tape prior to each use. It can be used as often as NVP causes a problem, and used from the onset of symptoms for at least 20 mins initially, or until sickness eases or stops.
As we all know, questionnaires are notorious for—getting lost, getting eaten by the dog, left in the rain, drawn on by the toddler, etc., but the women of Andover were very helpful, and the results followed.
150 audio-tapes were offered.
To date we have had 89 questionnaires returned fully completed, 25 fully completed but non-compliant. These returns, we felt couldn’t be in the final numbers, because other remedies had been tried at the same time, i.e. homeopathic remedies, herbal teas, Travel bands etc. 37 questionnaires were not completed for a variety of reasons, the most frequent being that the tape was never used.
PIE CHART
The results have been excellent. Originally we thought if it worked for 30% of women that would be amazing. At the time of writing this article it is 90%. The results show that of the 89 women that used the tape appropriately only 3 had no benefit at all. The results to date show that the greatest percentage of efficacy is produced by the 4th day. Some women found no effect the first time, but subsequent usage produced increasingly good effects.
PERCENTAGE CHART.
The whole mood of the study has changed. Originally we were all very cynical, and felt that some of the ecstatic responses we received must be due to other reasons but gradually the women’s comments, testimonials, ticks in the box, have made us take it all more seriously. We are now disappointed if the tape fails to ease the symptoms, and try and ensure that the tape is being used in the most beneficial way. A full list of the results, and the testimonials can be seen on the web site: http://www.andoverbirthcentre.org.
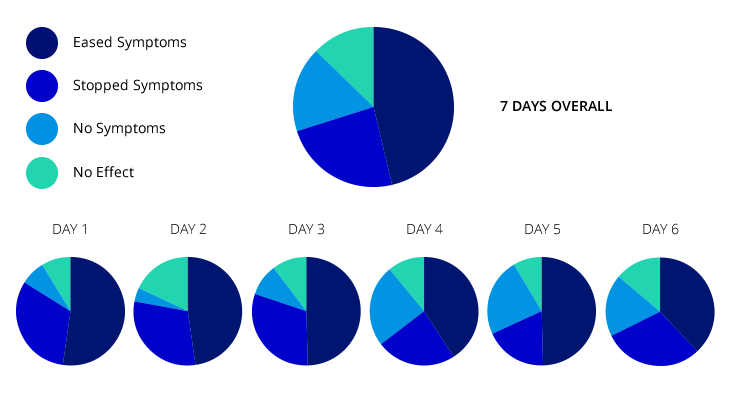
References
Whitehead SA, Andrews PLR, Chamberlain GVP. Characterisation of nausea and vomiting in early pregnancy: a survey of 1000 women. Journal of obstetrics and gynaecology 1992;12:364-9 Lacroix R, Easton E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Birth 199;182(4):931-7 Lindsay P. Vomiting.In: Sweet B. Mayes Midwifery, 12th edition.London: Bailliere Tindall,1997 Web-based Reference.
- Veyrat-Follet C; Farinotti R; Palmer JL.. Physiology of Chemotherapy-induced emesis and antiemetic therapy. Medline Indexing Date :199706. www.medscape.com/medline.
In relation to the information gained from clinical trials with Cancer patients;
The recent clinical using nevasic trial using Nevasic® to assist in dealing with the symptoms of nausea and vomiting in relation to chemotherapy treatment.
The clinical trial was commissioned by National Cancer Research UK, run and managed by “CECO” – a research authority.
Research was instigated by Professor Alex Molassiotis, Professor of Cancer and Supportive Care at the University of Manchester and funding was provided by an independent pharmaceutical company.
The results of this trial are still under review for publication – however there are some startling and pleasing facts to have been passed to us as a result of the research;
Protocol
The protocol for the trial was prepared by staff of the university under the guidance of the department CECO.
To maintain independence and impartiality DAVAL were not involved in this process or during the course of the trial.
While the results of the trial were not what we were hoping for – they were also not what we were expecting.
This trial highlighted the difficulty in setting up and running a trial of this nature – as the trial was run in Iran.
The key findings highlighted as “commentable” by the trial management are to be construed as positive in that while it was stated by patients that little or no benefit derived from the use of our programme – the amount of follow up care and treatment demanded by that same group was significantly less. This was described by the management as something that could have significant implications from a “cost” perspective – rather than looking purely at the “quality of life” perspective.
A very pleasing note was their comment regarding the side effects…
Key findings are;
- No significant differences in nausea/vomiting levels among three groups.
- Significant LESS use of antiemetics post-chemo (PRN) in Nevasic group; good and solid result as it has cost implications and less side effects.
- Borderline non-significant (p=0.06) better Global Health Status in Nevasic group.
- However, shortcomings of data: Nevasic pts had more CINV risk factors and less use of aprepitant (a key primary antiemetic), which may partly explain finding similar levels of CINV in Nevasic and control.
Some good and solid results and some not so good. The imbalance in the study groups in aprepitant use and risk factors makes replication of the study necessary before final conclusions, and possibly use of PRN antiemetics and health care costs being the key outcome to measure.
The social group using Nevasic during their treatment were patients who had undergone mastectomy procedures. We are informed the next trial (under planning now) will be a different niche user group.
Access to the protocol document and chapter 9 interim results can be obtained – after permission is granted by the trial management – you may ask – but you may not receive…just yet…
6. Transparency of Authorship
Term: The designers of this Web site will seek to provide information in the clearest possible manner and provide contact addresses for visitors that seek further information or support. The Webmaster will display his/her E-mail address clearly throughout the Web site.
DAVAL Compliance:
We believe in complete transparency with regard to our product and its independent research. We include wherever and whenever possible (and appropriate) full disclosure of information relating to who has researched what – using our product – and we include wherever and whenever we can the full information covering that research.
We seek to provide this is the clearest possible manner – while at the same time providing direct access to us – in order that you may ask your own questions relating to the research and its generated data.
If you need support with any aspect of our web site a contact email link is placed at the bottom of every page in order that you can contact us – plus there is a contact form. We welcome your contact on any issue regarding the materials contained in this site.
7. Transparency of Sponsorship
Term: Support for this Web site will be clearly identified, including the identities of commercial and non-commercial organisations that have contributed funding, services or material for the site.
DAVAL Compliance:
This web site is provided by and funded by DAVAL Ltd. There are no external influences, sponsorship or funding for this web site at this time and if this position changes those facts will be posted here.
8. Honesty in advertising & editorial policy
Term: If advertising is a source of funding it will be clearly stated. A brief description of the advertising policy adopted by the Web site owners will be displayed on the site. Advertising and other promotional material will be presented to viewers in a manner and context that facilitates differentiation between it and the original material created by the institution operating the site.
DAVAL Compliance:
There are no third party advertising materials submitted to this site, or paid for advertising displayed within this site. This site promotes the use of a product that has been clinically trialled for the treatment of the symptoms of nausea and vomiting and is the advertising vehicle for that product.
In maintaining our web site with a 100% pure concentration on DAVAL and our own products we are able to assert that there will never be any grey area where information regarding another company or product or service will be confused for our own.
If we were to introduce any commercial element sponsored by a third party it would be identified as such – making clear the distinction in the resource and identity of the supplier involved.
